
Who Has The Highest Psr Rating Ever 2022, 2K MOBILE IS 10x BETTER THAN NBA 2K22! (NEW PARKS), 11.63 MB, 08:28, 130,534, COLETHEMAN, 2021-12-17T00:57:12.000000Z, 19, Yamaha PSR-E373 keyboard review – TrendRadars, www.trendradars.com, 590 x 410, png, , 5, who-has-the-highest-psr-rating-ever-2022, KAMPION
Solubility of pb(io 3) 2 in water. At equilibrium the solution is saturated with pb(io. All group i and ammonium (nh4+) compounds are water soluble. All lead (pb) compounds are water insoluble except pb (no3)2.
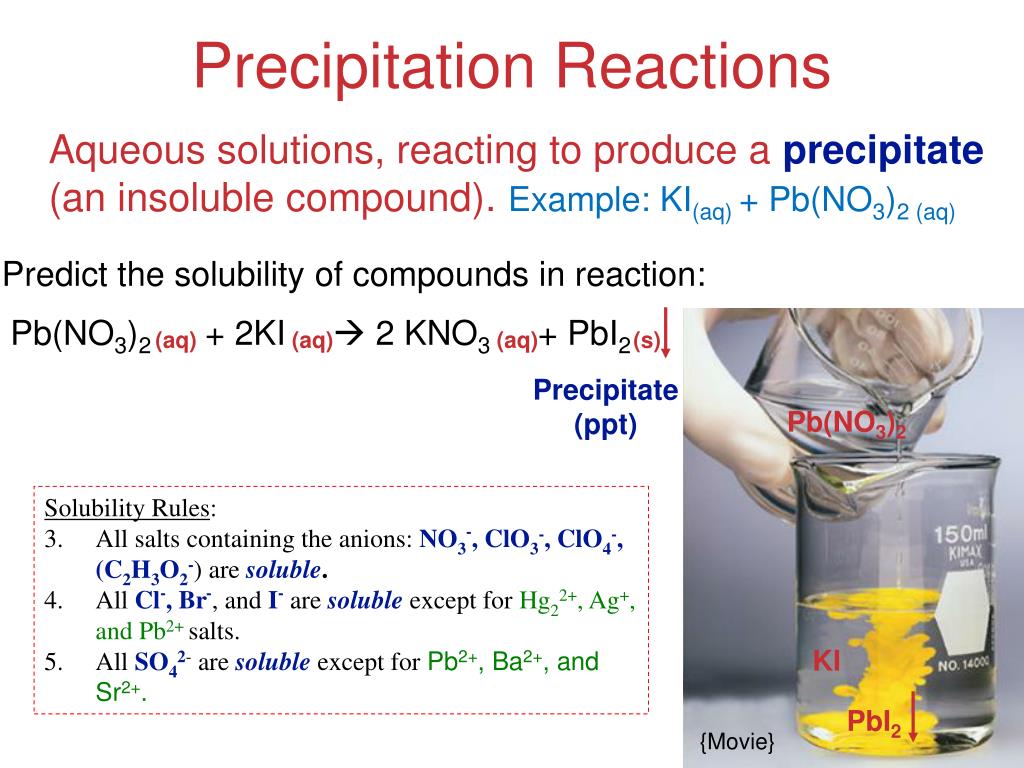
Predict whether the compounds are soluble or insoluble in water. Soluble insoluble kno3 cucl2 agbr. In this reaction, two soluble products, pb(no3)2 and ki, combine to form one soluble product, kno3, and one insoluble product, pbi2. This is a precipitation reaction, and pbi2 is the precipitate. Aq = aqueous, or soluble in water. Is pbi2 polar or nonpolar? Is pb(no3)2 (lead (ii) nitrate) soluble or insoluble in water? The answer is that it is soluble in water. It is an ionic compound which readily dissociates i.
Is Pb(NO3)2 Soluble or Insoluble in Water? - YouTube

4 to post 1

Acids, Bases and Salts (Chemistry 'O' level)
Solved: When Aqueous Solutions Of Fe2(SO4)3 (aq) And Pb(NO... | Chegg.com
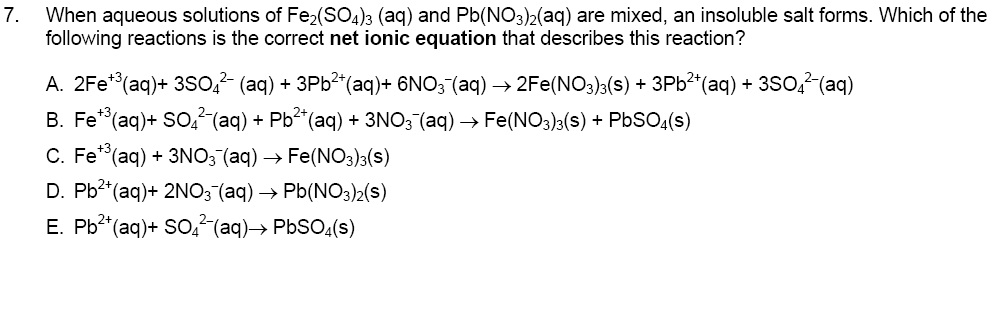
Solved: 3. Indicate Whether Each Of The Following Salts Is... | Chegg.com

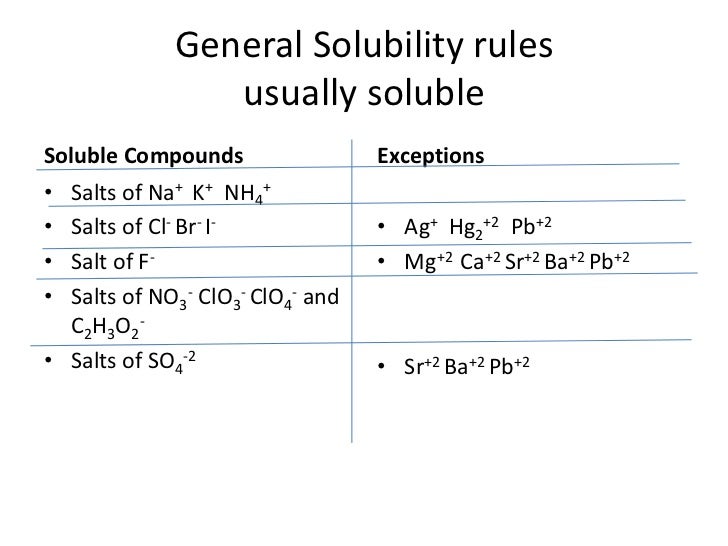
Solubility rules usually soluble
Solution & Solubility
Some solubility rules for ionic compounds in water are shown

PPT - Unit # 4: Aqueous Reactions and Solution Stoichiometry PowerPoint
PPT - Chapter 5 Molecular View of Reactions in Aqueous Solutions Part I
Komentar
Posting Komentar